Why Buffer Solution Is Used in Edta Titration
EDTA can be standardized against many reagents be it metallic magnesium calcium carbonate metallic bismuth and so on. This is because all the reactions between the metal ions and EDTA are pH-dependent.

Solved 3 For The Complexometric Titrations In This Chegg Com
This is because all the reactions between the metal ions and EDTA are pH-dependent.

. Why is pH 10 maintained in complexometric titration. Change in pH may lead to. Note that EDTA solution can be prepared without a need for standardization as EDTA itself can be.
A buffer solution is used in EDTA titration because it resists the change in pH. Carrying out the reaction in a basic buffer solution removes the H as it is formed. Calcium Analysis by EDTA Titration PRESTUDY 1.
Answer 1 of 6. A buffer solution is used in EDTA titration because it resists the change in pH. The titration with EDTA is the most true method as it defines the parameter hardness.
Because all reactions between metal ions and EDTA are pH dependent. Subsequently one may also ask why is buffer used in EDTA titrations. Stay tuned with BYJUS to learn more about other concepts such as titration.
It is necessary to keep the pH at about 10 for two reasons. A buffer solution is used in EDTA titration because it resists the change in pH. This is because all the reactions between the metal ions and EDTA are pH-dependent.
Which is the best method for hardness determination and why. Because all reactions between metal ions and EDTA are pH dependent. What is the main function of a buffer solution.
The principal reason that EDTA is utilized so broadly in the normalization of metal cation solution is that the development steady for most metal cation-EDTA complexes is high. The buffer solution is used in EDTA titration because it avoids the change in pH so that reactions take place between metal ions and EDTA. This buffer is also called Ammoniacal Buffer Dissolve 90 g of ammonium chloride in 375 mL of 2830 ammonium hydroxide and dilute to 500 mL with water.
For best results it is good to standardize EDTA solution against the same cation and using the same method as will be later used during sample analysis. EDTA is Ethydiaminotetraacetic acid. Why Is pH 10 Buffer Used In EDTA Titration.
During titration Eriochrome Black T is used as indicator. The Buffer solution is used to resist the change in pH. A buffer is a solution that can resist pH change upon the addition of an acidic or basic components.
Change in pH may lead to the improper reaction between Metal ion and EDTA. A 2500 mL aliquot of the solution required 2425 mL of an EDTA solution for titration to the Eriochrome Black T end point. As we need Y4-.
Why you use pH 10 buffer in EDTA titration. Download the pH 10 ammonia buffer solution preparation file. With increasing the pH each hydrogen ion in the carboxyl groups of EDTA will start to dissociate.
The titration which involves the formation of complexes is known as Complexometric titration. EDTA is often used as the disodium salt Na2H2Y. Why buffer is added in hardness determination by EDTA.
EDTA is commonly used a complexing agent which make a complex with metal ions. A buffer solution is used in EDTA titration because it resists the change in pH. It has four carboxyl groups and two amine groups that can act as electron pair donors Lewis bases.
Concentrated pH 10 ammonia buffer. Change in pH may lead to. Why buffer is added in hardness determination by.
The Buffer solution is used to resist the change in pH. Because all reactions between metal ions and EDTA are pH dependent. This is because all the reactions between the metal ions and EDTA are pH-dependent.
What is the role of ammonia buffer. Subsequently question is why buffer is added in hardness determination by EDTA. This is because all the reactions between the metal ions and EDTA are pH-dependent.
This is a concentrated buffer of high ionic strength added in small amount to titrated samples. How many moles of CaCO3 were used. This is because all the reactions between the metal ions and EDTA are pH-dependent.
The Buffer solution is used to resist the change in pH. Why do we use buffer solution in EDTA titration. PH 10 buffer is used in EDTA titration because in EDTA Y4- is predominant and we want Y4- to react with the metal ions that are present in the.
Change in pH may lead to the improper reaction between Metal ion and EDTA. After opening the file. EDTA is commonly used a complexing agent which make a complex with metal ions.
The Buffer solution is used to resist the change in pH. Always remember that a buffer is added to the solution that undergoes titration in. Because all reactions between metal ions and EDTA are pH dependent.
A buffer solution is used in EDTA titration because it resists the change in pH. A buffer solution is used in EDTA titration because it resists the change in pH. Open it with the free trial version of the buffer calculator.
Because all reactions between metal ions and EDTA are pH dependent. In some titrations it is best to avoid any sharp pH changesexcept in acid-base titrations of. Above pH 10 Y4- is predominant.
PH 10 buffer is used in EDTA titration because in EDTA Y4- is predominant and we want Y4- to react with the metal ions that are present in the titration solution. Both the metal ions and EDTA are pH- dependent. To prepare the recipe for a needed volume of the solution use ChemBuddy Buffer Maker program.
Ethylene diamine tetra acetic acid. EDTA is insoluble in water at low pH because H4Y is predominant in that pH less than 2. The Buffer solution is used to resist the change in pH.
This is because all the reactions between the metal ions and EDTA are pH-dependent. This can be achieved by using a pH 10 buffer. Which buffer solution is used in EDTA.
Control of pH is important since the H ion plays an important role in chelation. This is because all the reactions between the metal ions and EDTA are pH-dependent. A all reactions between metal ions and EDTA are pH dependent and for divalent ions.
During titration Eriochrome Black T is used as indicator. Why must use buffer solution in titration involving EDTA. In the EDTA titration metal ion indicator is used to detect changes of pM.
EDTA often written as H4Y is a common ligand in complexometric titrations. EDTA is commonly used a complexing agent which make a complex with metal ions. Which buffer solution is used in hardness determination.
This buffer is also called Ammoniacal Buffer Dissolve 90 g of ammonium chloride in 375 mL of 2830 ammonium hydroxide and dilute to 500 mL with water. We have to know that the reason why the buffer is used in EDTA titration is because it avoids the pH change. A 04505 g sample of CaCO3 was dissolved in HCl and the resulting solution was diluted to 2500 mL in a volumetric flask.

Complexometric Determination Of Magnesium Using Edta By Pablo Ortiz

Total Hardness Test Kit Hi38033
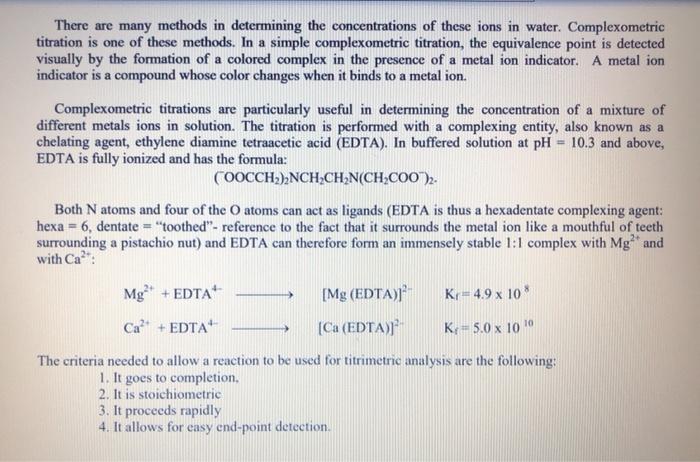
Solved Determining The Hardness Of Different Water Samples Chegg Com

Complexometric Titrations Ppt Video Online Download

Practical Eng Chemistry Hardness By Edta Fundamental Of Computer Studocu

Why Buffer Solution Is Used In Edta Titration

Jual Hach 42432 Hardness 1 Buffer Solution 100 Ml Mdb Kota Bekasi Onestop Laboratory Tokopedia

Jual Hach 42449 Hardness 1 Buffer Solution 500 Ml Cv Andalan Prima Sejahtera Bekasi Bekasi Indotrading

Jual Hach 42453 Hardness 1 Buffer Solution 1 L Cv Andalan Prima Sejahtera Bekasi Bekasi Indotrading

Complexometric Titrations Ppt Video Online Download

Ph 13 00 500ml Buffer Solution At 25c

Buffer Solution Definition Examples And Preparation

Hardness Of Water By Edta Titration

Determining Total Hardness Of Supply Water By Edta Buffer Solution Conical Flask Flask

Doc Complexometry Determination Hardness Of Water Ianatus Syarifah Academia Edu

Jual Hach 42432 Hardness 1 Buffer Solution 100 Ml Mdb Product Di Lapak Onestop Laboratory Bukalapak



Comments
Post a Comment